
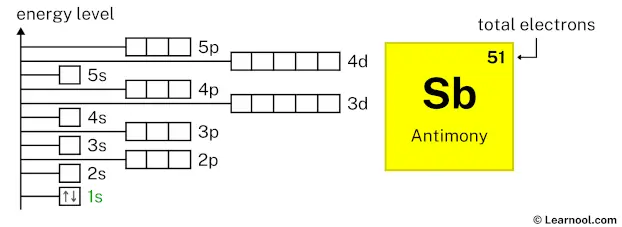
Is one of the highest-energy electrons of iodine, #"I"#, which is a nonmetal. Likewise, an electron described by this set of quantum numbers Is one of the highest-energy electrons of antimony, #"Sb"#, which is a metalloid. Carbon is situated in Group 14th and has an atomic number of 6. The number of valence electrons available for Carbon atoms is 4. The electron configuration for the Carbide ion (C 4-) is 1s 2 2s 2 2p 6. The shorthand electron configuration for Carbon is He 2s 2 2p 2. Is the highest-energy electron of indium, #"Id"#, which is a metal.Īn electron described by this set of quantum numbers And for the excited state, it is 1s 2 2s 1 2p 3. The problem with being a part of the p-block is that this block contains metals, metalloids, and nonmetals.įor example, an electron described by the following four quantum numbers If by "type of element" you mean metal, metalloid, transition metal, or nonmetal, then the information provided to you is not enough to allow you to give a definitive answer. Now, atoms that have their highest-energy electrons located in the p-subshell will be part of the p-block.
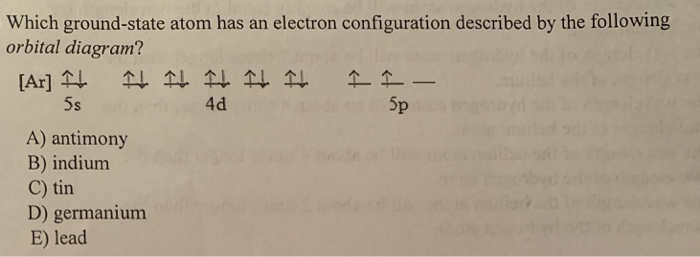
Now, take a look at how the periodic table can be split up into (at least) four distinct blocks #l=1 -># the electron is located in the p-subshell.#n = 5 -># the electron is located on the fifth energy level.The problem provides you the value of the principal quantum number, #n#, which tells you the energy level on which the electron resides, and the angular momentum quantum number, #l#, which tells you the subshell in which the electron is located. If by "type of element" you mean which block it belongs to, then all you have to do here is interpret the values of two quantum numbers provided to you.Īs you know, each electron that's part of an atom is characterized by four unique quantum numbers.


 0 kommentar(er)
0 kommentar(er)
